Equality, Diversity and Inclusion Strategy

Acronyms
We have tried to minimise acronyms in this strategy. The ones we have used can be found below:
BRC: Biomedical Research Centre
CRF: Clinical Research Facility
EDI: Equality, Diversity, and Inclusion
ERAF: Equality Risk Assessment Form
KCH: King’s College Hospital
KCL: King’s College London
NIHR: National Institute for Health and Care Research
PPI/E: Patient and Public Involvement/Engagement
SLaM: South London and the Maudsley NHS Foundation Trust
At the end of each section of the strategy there will be a blue hyperlink which will bring you back to this ‘acronyms’ section where you can view the acronyms written out in full.
Definitions
Key definitions from the National Institute for Health and Care Research (NIHR) are listed:
Clinical Trial: an experiment to compare the effects of two or more medicines, treatments, or procedures. ‘Clinical trial’ is an umbrella term for a variety of different experiments using different methods.
Equality: ensuring that everyone is given equal access to resources and opportunities to use their skills and talents. Taking a systems approach to what we do and how we do it and identifying and removing long standing, structural barriers to success.
Equity: trying to understand and give people what they need to achieve their potential; promoting notions of fairness, justice, entitlements, and rights.
Diversity: being reflective of the wider community. Having a diverse community, with people from a broad range of backgrounds represented in all areas and at all levels.
Inclusion: an approach where groups or individuals with different backgrounds are welcomed, culturally and socially accepted, and treated equally. Engaging with each person as an individual. A sense of belonging that is respectful of people for who they are.
Intersectionality: A framework that acknowledges that all people have unique experiences of discrimination and disadvantage exacerbated by the overlap of multiple social identities.
Public members: include patients, potential patients, carers, and people who use health and social care services as well as people from organisations that represent people who use services.
Research: discovering new knowledge that could lead to changes in treatments, policies, or care.
Introduction and our commitment and vision
This strategy outlines our ambition, aim, principles, and goals for Equality, Diversity, and Inclusion (EDI) at the King’s Clinical Research Facility (CRF). We aspire to be a diverse and inclusive research environment. We want the research undertaken at the King’s CRF, and the research teams who use our facilities, to reflect this aspiration.
We are aware that our equality, diversity, and inclusion journey is at an early stage. Whilst we are currently meeting all requirements, there is scope for us to adopt new practices, and for us to strengthen a culture where EDI sits front and centre. We recognise that on the EDI Maturity Model [1], we are at the ‘compliance’ stage; we greatly look forward to moving through to the next stages of discovery and commitment.
Our work at the King’s CRF is funded substantially by the NIHR, and we use their definitions of equality, diversity, and inclusion. Equality is ensuring that everyone is given equal access to resources and opportunities to use their skills and talents. Diversity is being reflective of the wider community; having people from a broad range of backgrounds represented in all areas and at all levels. Inclusion is an approach where groups or individuals with different backgrounds are welcomed, culturally and socially accepted, and treated equally.
Vernā Myers, an inclusion strategist, succinctly summarises this in the following analogy. We have added, ‘belonging’ into the analogy, as we also want people at our Clinical Research Facility to feel free to be themselves.
Vernā MyersDiversity is being invited to the party.
Inclusion is being asked to dance.
Belonging is dancing like nobody is watching.
We want staff, researchers, and public members to read and use this document often. We understand that there will be a need for us to learn continually and actively. We will need to learn from our successes and failures, from others within and outside our sector, and from our public members and the wider community we serve. This ‘learning approach’ is clearly outlined in the NIHR EDI Strategy [2], and it will be required for long-term success when embedding EDI in the work of the King’s Clinical Research Facility.
[1] https://diversity.tapnetwork.ca/maturity-model1
[2] https://www.nihr.ac.uk/documents/equality-diversity-and-inclusion-strategy-2022-2027/31295
The King’s Clinical Research Facility
The King’s Clinical Research Facility is designed to support clinical trials on a broad range of topics including mental health and general medicine. These clinical trials may be sponsored by pharmaceutical companies (known as commercial trials) or sponsored by the NHS, Research Councils or Charities (known as non-commercial trials).
The King’s Clinical Research Facility is made up of four research areas listed below. They are all based physically at King’s College Hospital acting in partnership with South London and the Maudsley NHS Foundation Trust and King’s College Hospital.
- The Experimental Medicine Facility: this contains rooms set up with clinical equipment, such as the equipment needed to take blood or to give medication, and specialised rooms where volunteers and patients with particular conditions taking part in clinical trials can be interviewed.
- The Cell Therapy Unit: where human-cell and gene-based therapies can be produced.
- The Clinical Trials Facility: this contains ward beds and rooms where volunteers and patients with particular conditions taking part in clinical trials can be examined.
- The Imaging Facility: this contains a 3T magnetic resonance imaging scanner and we are expecting a second scanner to be installed in 2023.
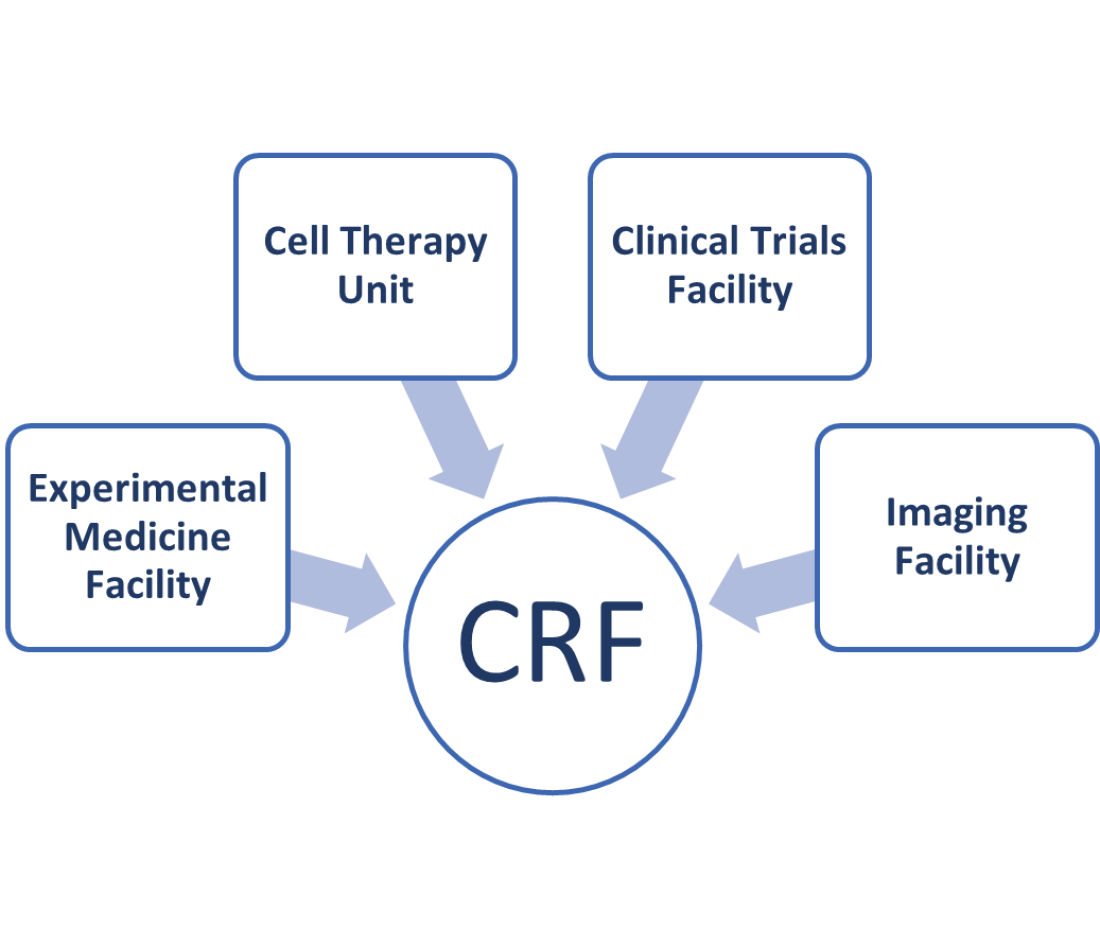
Figure 1: Research areas within the King’s Clinical Research Facility
The King’s Clinical Research Facility has clinical research and support staff to help research teams with their studies. Research teams from across the hospital undertaking commercial or non-commercial trials must apply to use the CRF and to work with our staff.
The wider King’s Clinical Research Facility Strategy outlines our aim to provide an excellent facility for the efficient and safe conduct of clinical trials. We hope these clinical trials will lead to developments that transform the lives of patients. We believe this aim can only be achieved by working alongside:
- Researchers from many different disciplines, locations, and backgrounds
- Staff, such as Research Nurses, Clinical Research Practitioners, and Administrators, from many different disciplines, locations, and backgrounds
- Public members from many different locations and backgrounds with varied work and lived experiences and interests
Our partners and collaborators
We receive support from many places. Our partners and collaborators have influenced this Equality, Diversity and Inclusion Strategy as follows:
King’s Health Partners
We receive academic support from King’s Health Partners [1]. King’s Health Partners is a collaboration between hospitals and universities in the Southeast London area. King’s Health Partners’ five-year plan centres on delivering better health for all. One way they plan to approach this is by developing and evaluating opportunities for black and minority ethnic staff and students. This Equality, Diversity and Inclusion Strategy supports King’s Health Partners’ approach.
King’s College Hospital
We are based in King’s College Hospital, and therefore we align ourselves with the hospital strategy, which is to be BOLD [2]:
- Consisting of Brilliant people
- Delivering Outstanding care
- Developing Leaders in research and education
- With Diversity, Equality, and Inclusion at the centre of it all
We plan to collaborate with colleagues from the King’s College Hospital equality, diversity, and inclusion team, and we also align ourselves with their first-ever ‘Roadmap to Inclusion’ [3]. This sets out some key actions including the introduction of EDI training and mentoring programmes and increasing the diversity of research teams. There is also a Research Health Inequalities Group, which is part of the hospital Health Inequalities Programme. The group has many priorities that fit with the scope of this Strategy. For example, the group will develop inclusive communications with patients to encourage their participation in research. We plan to work with the Research Health Inequalities Group as outlined in goal 3 in our action plan.
King’s College London
We are academically based in King’s College London. We plan to collaborate with colleagues from the King’s College London equality, diversity, and inclusion team. We would like to learn from them, particularly in relation to the Diversity Matters and Trans Matters training programmes they deliver [4]. We benefit from training that academic staff have available to them in these areas.
South London and the Maudsley (SLaM) and its Biomedical Research Centre (BRC):
We have significant interactions with SLaM and its BRC. The SLaM BRC has considerable expertise around equality, diversity and inclusion that we can leverage to the advantage of the King’s CRF.
NIHR
We receive funding from the NIHR. We are also supported by the NIHR’s EDI infrastructure, available resources, and guidance.
To note: We also received funding from the Wellcome Trust when we were first established. More information on our funders can be found here.
Public members
Our public members are key partners and collaborators. As more public members join us, their local community contacts and networks will be invaluable in helping us to build a diverse group. We want the group to be reflective of the South London population we serve.
[1] https://www.kingshealthpartners.org/
[2] https://www.kch.nhs.uk/about/our-strategy
[3] https://www.kch.nhs.uk/document/roadmap-to-inclusion-2022-2024/
[4] https://www.kcl.ac.uk/professional-services/diversity/edi-training-for-staff
Our ambition and aim for EDI
Our ambition
To support more inclusive research at the King’s Clinical Research Facility, which is undertaken by diverse research teams, and shaped by a diverse public involvement group.
Our aim
To raise awareness of the importance of EDI, and to ensure that appropriate resources and training are available and accessible to all staff at the King’s Clinical Research Facility.
Our ambition and aim are built on and in line with the NIHR’s EDI Strategy for 2022-2027 [1]. Their strategy outlines the need for people from diverse backgrounds in the research talent pipeline, and the need for appropriate training, resources, guidance, and support to embed EDI within research cultures.
[1] https://www.nihr.ac.uk/documents/equality-diversity-and-inclusion-strategy-2022-2027/31295
Our principles
At the King’s Clinical Research Facility we are:
Curious individuals
we take time to learn from, and about, people with different experiences and backgrounds than our own; difference is celebrated
Courageous individuals
we speak up and draw attention to behaviours or practices that need to be challenged; we learn from both success and failure
Capacity-building individuals
We value researchers, and staff at all stages of their careers and seek to support their career progression
Our equality, diversity, and inclusion principles are closely aligned with those detailed in the King’s CRF Public Involvement and Engagement Strategy (which are relationship-centred, respectful, and responsive involvement). We want to ensure that researchers, staff and public members’ diverse experiences, backgrounds and opinions are heard and respected and influence and enrich the work of the King’s Clinical Research Facility.

Figure 2: Our principles
Our goals
Goal 1: To collect diversity monitoring data, which will be published on a dedicated area of the King’s Clinical Research Facility public website
Goal 2: To develop inclusive communications that encourage diverse research teams and public members to work with the King’s Clinical Research Facility and to use our facilities
Goal 3: To engage and educate our staff through high quality training courses on pertinent EDI topics, including an understanding of bullying and harassment
Goal 4: Evaluate the impact of EDI activities within the King’s Clinical Research Facility
Our action plan
For goal 1, we plan to:
- Survey staff and public members about their personal characteristics (including age, gender, disability, sexual orientation, and ethnicity). Use guidance from Equality, Diversity, and Inclusion in Health and Science [1], the NIHR and the KCH EDI team in survey development.The KCH EDI team’s work on the ‘King’s Model’ to support ethnically diverse research recruitment will be particularly helpful. We will do this by the end of 2023, then collect data annually. (Responsible individuals: PPIE Lead, Communications Officer, and CRF Manager)Key outcomes: To address barriers relevant to underrepresented groups identified in the surveys, and to share learnings and work with the KCH EDI team.Success criteria: 2024 surveys demonstrate more diversity within staff and public members. Learnings are shared and working relationship strengthened with KCH EDI team and progress is shared from the ‘King’s Model’ work, and 2024 surveys are adapted as a result.
- Set up a dedicated EDI section on our website. Diversity monitoring data and reports to be hosted here. We will do this by mid 2024. (Responsible individuals: PPIE Lead and Communications Officer)Key outcomes: To develop a regularly updated and engaging EDI section of the website.Success criteria: EDI section of the website is live and regularly updated. Diversity reports are uploaded.
- Introduce question(s) on our application form that asks a) how the proposed research plans to address health inequalities b) About the diversity of research participants and the research team. We will do this by early 2023, then collect data annually. (Responsible individuals: PPIE Lead and Data Manager)Key outcomes: To review the diversity of participants and research teams, and to monitor health inequalities information within application forms.
Success criteria: Research teams are representative of local populations. Research teams consider and acknowledge how their research addresses health inequalities.
For goal 2, we plan to:
- Adapt the KCH Equality Risk Assessment Form to help us ensure communications, practices, events, and decision-making processes are fair and do not present barriers to participation or disadvantage any protected groups from participation. Intersectionality to be considered within these assessments. See Appendix 1. We will do this by the end of 2023. (Responsible individuals: PPIE Lead and Communications Officer)Key outcomes: To adapt a user-friendly Equality Risk Assessment Form which is stored on our electronic system (Q-Pulse) alongside other forms and policies. This system is accessible to all staff.Success criteria: Equality Risk Assessment Form is used, and strategies are put in place to mitigate against any issues.
- Review the accessibility of the King’s CRF website. Make use of the new NIHR guide to creating inclusive language and content, once this has been published. We will do this by early 2025. (Responsible individual: Communications Officer)Key outcomes: To review the simplicity of the content and the ability to zoom in on content, and to review the number of PDF documents (as these are not fully accessible to all screen readers).
Success criteria: The website is accessible, with a good readability score, and content appropriate for screen readers. - Participate in national networking around Equality, Diversity, and Inclusion to learn best practice and share resources (including around inclusive communications). We will do this by mid 2024. (Responsible individuals: PPIE Lead, Communications Officer, and CRF Manager)Success criteria:Working partnerships and relationships established with national EDI-forward organisations. Library of resources established.
For goal 3, we plan to:
- In collaboration with the KCH EDI team, introduce a training needs survey for our staff to understand what training and resources are required to build confidence in EDI. We will do this by the end of 2023, then annually. (Responsible individuals: PPIE Lead and Communications Officer)Key outcomes: To review the specific EDI training needs of our staff.Success criteria: Understanding of staff training needs used to develop plans for training sessions.
- In collaboration with the KCH EDI team, set-up a staff network/interest group on EDI. The Chair of the network/interest group will also sit on the Research Health Inequalities Group. We will do this by the end of 2024. (Responsible individual: CRF Manager)Key outcomes: Network to promote relevant opportunities, and to be involved in further EDI strategy work once this strategy has come to an end.Success criteria: Up to 5 staff members involved in the network/interest group. Regular staff network/interest group meetings arranged and attended (by at least 3 members). Learnings and resources are shared between the staff network/interest group and the Research Health Inequalities Group.
- Identify opportunities to join and promote relevant awareness days and weeks (e.g., Race Equality Week). Use the KCH EDI annual Inclusion Calendar for this. We will do this by early 2023, then annually. (Responsible individuals: PPIE Lead, Communications Officer)Key outcomes: Awareness days are announced in the team morning meeting and event invites cascaded to all staff.
Success criteria: Staff know about the awareness days and attend related events. KCH EDI annual Inclusion Calendar highlighted and shared with staff. - Participate in local networking, and promote events and training run e.g., by the KCH EDI team. We will do this by mid 2023, then ongoingly. (Responsible individuals: PPIE Lead, Communications Officer, and CRF Manager)Key outcomes: EDI added to the induction process with information about key contacts, and key events/training included.Success criteria: Staff know about the KCH EDI team and are kept informed of local events and training.
- Invite external diversity consultancy, such as EW Group [2], to deliver relevant in-person or e-learning training dependent on survey results (possible topics include e.g., unconscious bias, active bystanders, bullying, and harassment). We will do this by the end of 2023, then annually. (Responsible individuals: PPIE Lead and CRF Manager)Key outcomes: To offer relevant and high-quality training for all staff to improve their confidence and competence in EDI.
Success criteria: EDI training is held annually and attended by >90% of CRF staff.
For goal 4, we plan to:
- Publish diversity monitoring data and relevant reports. We will do this by early 2024, then annually. (Responsible individuals: PPIE Lead and Communications Officer)Success criteria: Diversity reports are uploaded onto the EDI section of the website. Reports and learnings are shared with all our staff via email.
- Publish Equality Risk Assessment Forms. We will do this by the end of 2023, then annually. (Responsible individuals: PPIE Lead and Communications Officer)Success criteria: Equality Risk Assessment Forms are uploaded onto the EDI section of the website. Assessments and learnings are shared with all staff via email.
- Monitor and reflect on progress with specific EDI activities and events through use of evaluation forms. We will do this annually. (Responsible individual: PPIE Lead)Key outcomes: Run evaluations of each activity and event held
Success criteria: Evaluations demonstrate that activities and events are successful. Improvements made to subsequent events based on feedback. Learnings are compiled and reported.
Whilst this strategy does not cover Patient and Public Involvement (PPI) in depth, there is overlap between our plans for both EDI and PPI. For instance, in our PPI strategy, we outline how we plan to:
- Engage with education and young people: we will continue to work with local schools to offer work experience and/or open days for GCSE and A-level students. We will also offer internships for young adults with a learning disability and autism or both via partnering with DFN Project SEARCH [3]. We will do this by early 2023, then annually. Elka Giemza, CRF Manager, will be responsible for maintaining the working relationship with DFN Project Search.
Our roadmap
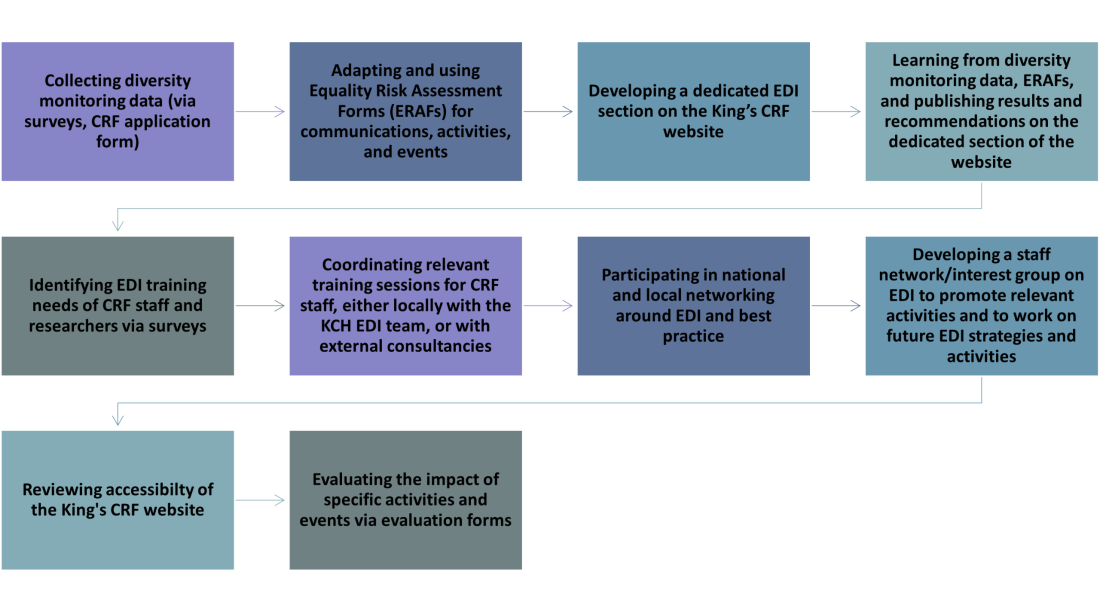
Resources and infrastructure supporting our strategy
Our strategy development and delivery will be supported by:
Elka Giemza, CRF Manager – with overall responsibility for putting this strategy into practice
PPIE Lead – with responsibility for ensuring effective consideration of EDI principles throughout the CRF’s activities
Our partners and collaborators at King’s College Hospital, King’s College London, South London and the Maudsley and its Biomedical Research Centre, the NIHR, and our public members will also provide support and guidance in the EDI work undertaken and outlined in this strategy.
There are funds to support EDI activities, such as training delivered by external organisations and consultancies as outlined in goal 3.
As part of this strategy, we are also proposing the following key structure to support EDI at the King’s Clinical Research Facility:
Staff Equality, Diversity, and Inclusion Group: a small group made up of staff members will develop and plan EDI-related activities and will review progress made against the strategy goals regularly as outlined in goal 3. Members will also help the CRF Manager and PPIE lead to explore how EDI might be strengthened more generally.
Our governance structures
We will:
- Add any EDI considerations as a standing agenda item in monthly management board meetings.
- Designate up to 4 EDI champions among the CRF research nurses and clinical research practitioners. The EDI champions will be an additional point of contact and support for staff who would like to know more about planned activities, resources, and guidance.
For reporting purposes, our PPIE Lead is accountable to Elka Giemza, CRF Manager. The CRF Manager is accountable to Professor Peter Goadsby, the Director of the CRF.
Evaluating our strategy
We will:
- Review progress with our strategy during regular meetings (with the EDI staff group once this has been established, and before this with the CRF Manager).
- Gather evaluation forms, Equality Risk Assessment Forms (See Appendix 1), and ongoing feedback on EDI activities and events as part of goals 2 and 4. This will inform the next 3 years of the strategy.
- Towards the end of the strategy obtain feedback via a survey from CRF staff, researchers, research participants and public members about progress made against each goal. This will inform the next 3 years of the strategy.
Acknowledgements
We would like to thank the following staff members who reviewed and improved this strategy:
- Professor Peter Goadsby, Director, King’s Clinical Research Facility
- Safiya Hassan, Clinical Research Practitioner, King’s Clinical Research Facility
- Simon O’Donoghue, Head of EDI (Patients and Communities), King’s College Hospital
- Amelia Te, Team Lead, King’s Clinical Research Facility
Contact
To find out more about equality, diversity, and inclusion at the King’s Clinical Research, please email our PPIE Lead.
Appendix 1. Equality Risk Assessment Form
This example of an Equality Risk Assessment Form was developed by the KCH EDI Team.
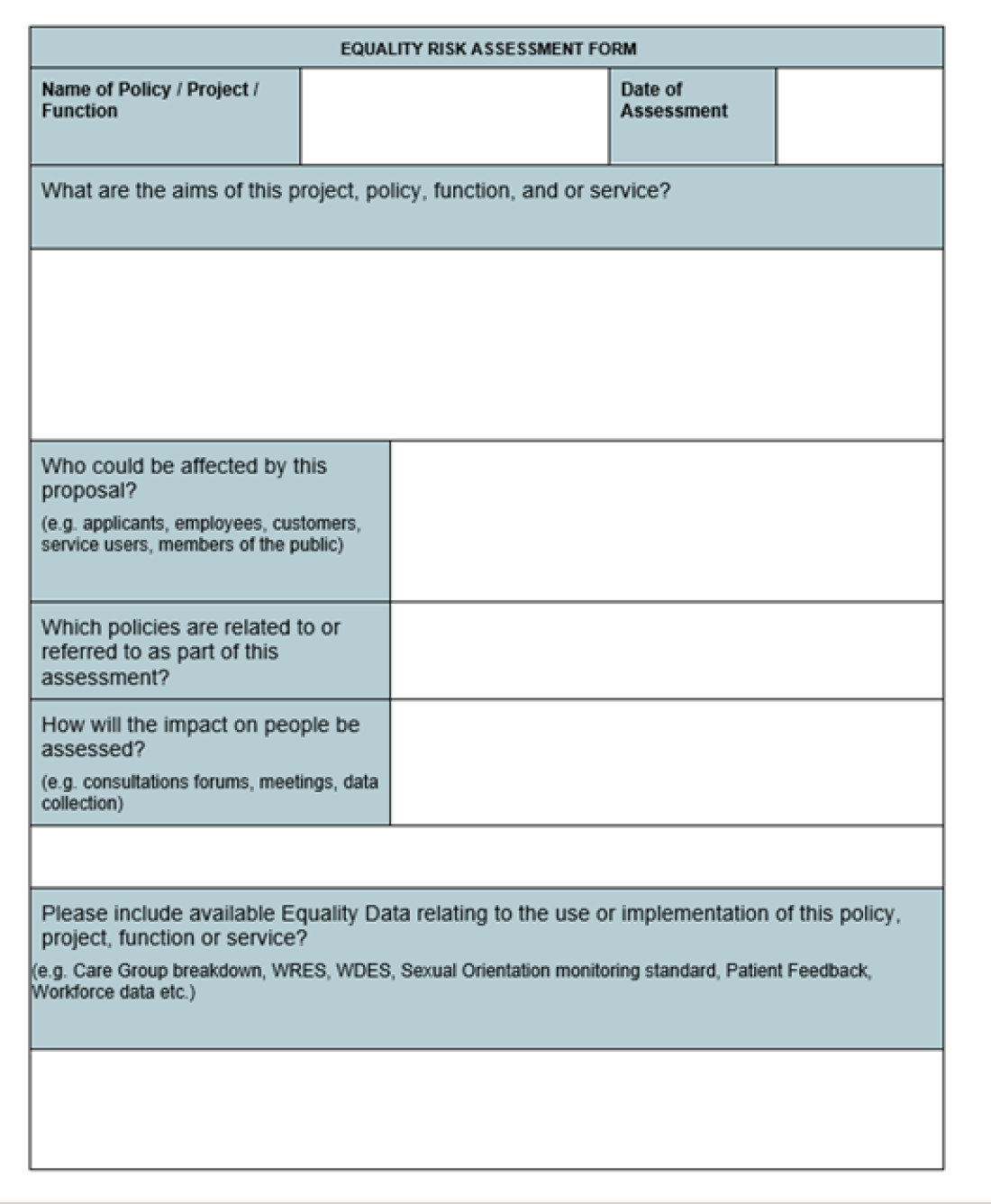
Equality Risk Assessment Form example snapshot
